1-(Chloromethyl)-2-Vinylbenzene as a Novel Polymeric Chain Extender
Polymer chain extenders are essential for modifying and processing polymers, improving their material properties. One key chain extender is 1-(Chloromethyl)-2-Vinylbenzene, also known as O-Chloromethyl styrene, produced by UniVOOK Chemical. This compound serves as an initiator for the polymerization of various copolymers and as a bifunctional styrene monomer in organic synthesis. The Uni-Extra series from UniVOOK includes multifunctional polymeric chain extenders with epoxy groups, which are widely used to modify polycondensation polymers. These extenders improve both the material and processing properties of polymers by increasing molecular weight and melt strength. This is achieved through controlled chemical reactions between the epoxy groups and functional groups such as -COOH, -OH, and -NH₂, ultimately enhancing material properties, processing ease, and fluidity.
Chemical Properties and Synthesis
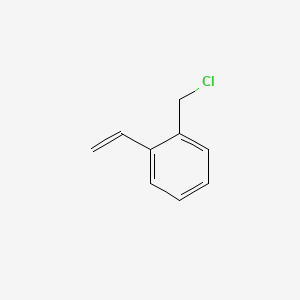
Detailed Chemical Structure of 1-(Chloromethyl)-2-Vinylbenzene
1-(Chloromethyl)-2-Vinylbenzene has a specific chemical structure. It consists of a benzene ring with two substituents. One substituent is a chloromethyl group (-CH₂Cl). The other is a vinyl group (-CH=CH₂). The chloromethyl group is attached to the first carbon of the benzene ring. The vinyl group is attached to the second carbon. This structure makes the compound bifunctional. It can react at both the chloromethyl and vinyl sites. The presence of the chlorine atom makes it reactive. The vinyl group allows it to participate in polymerization reactions. This dual functionality is key to its role as a chain extender. The structure also provides sites for bonding with polymer chains. This helps in linking polymer molecules together.
Synthetic Methods for Producing the Compound
There are several methods to synthesize 1-(Chloromethyl)-2-Vinylbenzene. One common method starts with styrene. Styrene undergoes chloromethylation. This involves adding a chloromethyl group to the styrene molecule. Typically, formaldehyde and hydrochloric acid are used in this reaction. The process adds the chloromethyl group to the benzene ring. Another method involves the direct reaction of chloromethyl chloride with styrene. This reaction also introduces the chloromethyl group to the vinylbenzene structure. The synthesis conditions, such as temperature and catalysts, are carefully controlled. This ensures the purity and yield of the final product. Both methods aim to produce 1-(Chloromethyl)-2-Vinylbenzene with high efficiency. Proper synthesis is crucial for its effectiveness as a chain extender.
Characterization of the Synthesized Compound
After synthesis, the compound is characterized to confirm its structure and purity. Spectroscopy is commonly used for this purpose. Infrared (IR) spectroscopy identifies functional groups in the molecule. The presence of chloromethyl and vinyl groups can be confirmed using IR spectra. Nuclear Magnetic Resonance (NMR) spectroscopy provides detailed information about the molecular structure. It shows the arrangement of atoms within the compound. Additionally, mass spectrometry can determine the molecular weight. Purity analysis is also conducted. Techniques like gas chromatography (GC) ensure that the compound is free from impurities. High purity is essential for its performance as a chain extender. Proper characterization ensures that the synthesized compound meets the required standards.
Mechanism of Chain Extension
How 1-(Chloromethyl)-2-Vinylbenzene Acts as a Chain Extender
1-(Chloromethyl)-2-Vinylbenzene acts as a chain extender by linking polymer chains together. The chloromethyl group reacts with functional groups in the polymer. This creates bonds between different polymer molecules. The vinyl group can also participate in polymerization. It allows the compound to integrate into the polymer backbone. This dual reactivity helps increase the molecular weight of the polymer. As a result, the polymer becomes stronger and more durable. The chain extender effectively bridges gaps between polymer chains. This enhances the overall structure of the polymer material.
Mechanistic Pathways for Polymer Chain Elongation
The chain extension process involves several steps. First, the chloromethyl group reacts with carboxyl (-COOH), hydroxyl (-OH), or amine (-NH₂) groups in the polymer. This forms a covalent bond, linking two polymer chains. Next, the vinyl group undergoes free radical polymerization. This reaction connects additional polymer chains to the existing structure. The overall effect is the elongation of the polymer chains. This increases the molecular weight and improves the mechanical properties of the polymer. The mechanism ensures that the polymer chains are effectively interconnected. This results in a more robust and stable polymer material.
The Role of the Chloromethyl Group and Vinyl Group in Polymerization
Both the chloromethyl and vinyl groups play crucial roles in polymerization. The chloromethyl group provides sites for bonding with functional groups in the polymer. This helps in linking polymer chains together. The vinyl group is reactive and can participate in free radical polymerization. This allows the compound to integrate into the polymer backbone. Together, these groups ensure that the chain extender effectively connects and strengthens the polymer chains. The dual functionality enhances the efficiency of the chain extension process. This leads to polymers with improved properties.
Polymerization and Processing with 1-(Chloromethyl)-2-Vinylbenzene
Experimental Setup for Polymerization
Polymerization experiments with 1-(Chloromethyl)-2-Vinylbenzene require careful setup. First, the polymer base is prepared. This could be a polyester, polyamide, or another suitable polymer. The chain extender is then added to the polymer base. The mixture is heated to initiate the reaction. A catalyst may be used to speed up the polymerization process. The temperature and time are controlled to ensure proper reaction. During polymerization, the compound reacts with the polymer chains. This links them together, increasing the molecular weight. After the reaction, the polymer is cooled and processed into the desired form. This setup ensures that the chain extension occurs efficiently.
Effect on Molecular Weight, Viscosity, and Polymer Structure
Using 1-(Chloromethyl)-2-Vinylbenzene as a chain extender significantly affects the polymer properties. The molecular weight of the polymer increases. Higher molecular weight leads to stronger and more durable materials. The viscosity of the polymer melt also increases. This makes the polymer more resistant to flow, which is beneficial in processing. The polymer structure becomes more interconnected. This enhances the mechanical strength and thermal stability of the polymer. Overall, the use of this chain extender improves the quality and performance of the polymer.
Comparison with Other Conventional Chain Extenders
When compared to conventional chain extenders, 1-(Chloromethyl)-2-Vinylbenzene offers several advantages. Traditional extenders like diols and diamines may require higher amounts to achieve the same effect. In contrast, 1-(Chloromethyl)-2-Vinylbenzene is more efficient. It provides better control over the molecular weight increase. Additionally, it enhances both mechanical and thermal properties more effectively. The dual functionality of this compound allows for more robust polymer structures. This makes it a superior choice for improving polymer performance. Furthermore, it can be used with a wider range of polymers. This versatility makes it valuable in various applications.
Properties of Polymers Modified with 1-(Chloromethyl)-2-Vinylbenzene
Thermal Properties
Polymers modified with 1-(Chloromethyl)-2-Vinylbenzene show improved thermal properties. The melting temperature of the polymer increases. This means the polymer can withstand higher temperatures without melting. The glass transition temperature also rises. This enhances the polymer’s resistance to deformation under heat. These thermal improvements make the polymers suitable for applications that involve high temperatures. They are more stable and maintain their properties better in such environments.
Mechanical Properties
The mechanical properties of polymers are significantly enhanced. The tensile strength of the polymer increases. This makes the material stronger and less likely to break under stress. The elongation at break also improves. This means the polymer can stretch more before breaking. These properties are important for applications that require durable and flexible materials. The enhanced mechanical strength ensures that the polymer can withstand demanding conditions.
Chemical Resistance and Durability
Polymers modified with 1-(Chloromethyl)-2-Vinylbenzene exhibit better chemical resistance. They are more resistant to degradation by chemicals. This makes them suitable for use in harsh chemical environments. The durability of the polymers is also increased. They have a longer lifespan and maintain their properties over time. This reduces the need for frequent replacements and maintenance. Improved chemical resistance and durability are crucial for industrial applications where materials are exposed to various chemicals.
Morphological Changes in the Polymer Structure
The addition of 1-(Chloromethyl)-2-Vinylbenzene causes notable morphological changes in the polymer structure. The polymer chains become more interconnected. This leads to a more uniform and stable structure. The increased molecular weight results in a denser polymer network. This enhanced network improves the overall strength and stability of the polymer. Additionally, the improved fluidity of the polymer melt allows for better processing and molding. These morphological changes contribute to the superior properties of the modified polymers.
Applications of the Modified Polymers
Potential Industrial and Commercial Applications
Polymers modified with 1-(Chloromethyl)-2-Vinylbenzene have a wide range of applications. In the automotive industry, they are used for making strong and lightweight parts. This improves fuel efficiency and vehicle performance. In the packaging industry, these polymers provide better durability and chemical resistance. This ensures the safe storage of goods. They are also used in the construction sector for making durable building materials. Additionally, these modified polymers are suitable for electronics due to their thermal stability. They can be used in insulating materials and components that require high temperature resistance.
Advantages of Using 1-(Chloromethyl)-2-Vinylbenzene Over Other Chain Extenders
Using 1-(Chloromethyl)-2-Vinylbenzene offers several advantages. It provides a higher increase in molecular weight with smaller amounts compared to other extenders. This makes it cost-effective. The dual functionality allows for better control over the polymer structure. This results in polymers with superior mechanical and thermal properties. Additionally, it can be used with a variety of polymers, making it versatile. The enhanced chemical resistance and durability extend the lifespan of the products made from these polymers. Overall, 1-(Chloromethyl)-2-Vinylbenzene is a more efficient and effective chain extender, leading to higher quality polymer materials.
Closing Thoughts
1-(Chloromethyl)-2-Vinylbenzene serves as a highly effective polymer chain extender, enhancing the strength and durability of polymers. By increasing molecular weight, it improves both thermal and mechanical properties. Compared to many conventional chain extenders, it demonstrates superior performance. This makes it valuable across diverse industries such as automotive, packaging, and construction. Its versatility allows compatibility with a wide range of polymers, offering substantial advantages in the production of high-performance polymer materials.
Access Our Product Catalog and More to Discover High-Performance Chemicals Tailored to Your Business Needs