Synthesis and Characterization of Dimethylanilinium Tetrakis(pentafluorophenyl)borate: Properties and Applications
Dimethylanilinium Tetrakis(pentafluorophenyl)borate is a specialized compound crucial in chemical synthesis and catalysis due to its unique properties. Understanding its synthesis and characterization is pivotal for exploring its applications across various chemical processes. Featuring a complex structure that combines dimethylanilinium cation with tetrakis(pentafluorophenyl)borate anion, it exhibits notable stability and reactivity. UniVOOK Chemical supplies high-performance Dimethylanilinium Tetrakis(pentafluorophenyl)borate(CAS No. 118612-00-3) products known for their exceptional efficiency in catalytic applications. As catalyst materials are indispensable in numerous chemical processes, UniVOOK has earned a distinctive reputation for reliability and performance in the industry.
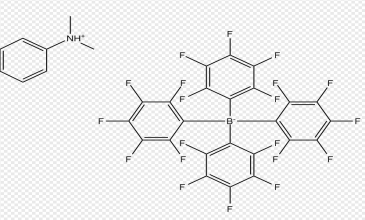
Synthesis Methods
Synthetic Procedures Overview
The synthesis of Dimethylanilinium Tetrakis(pentafluorophenyl)borate involves a meticulous multi-step process starting with the preparation of the dimethylanilinium precursor. This precursor undergoes controlled reaction conditions with tetrakis(pentafluorophenyl)borate to yield the target compound. Precise management of parameters such as temperature, pH, and reaction duration is essential to achieve high purity and yield.
Key Reagents and Conditions
Critical reagents include dimethylaniline, pentafluorophenylborate salts, and solvents like dichloromethane or acetonitrile. Inert atmospheres prevent oxidation, while catalysts or promoters aid the reaction. Maintaining anhydrous conditions and strict temperature control are pivotal to avoid side reactions and product degradation.
Challenges and Optimization Strategies
Synthesizing Dimethylanilinium Tetrakis(pentafluorophenyl)borate faces challenges, notably the sensitivity of pentafluorophenylborate to moisture and impurities. Optimizing parameters such as temperature, solvent selection, and reaction time enhances purity and yield. Efficient removal of by-products and unreacted materials is crucial for achieving the desired high-quality product.
Characterization Techniques
Spectroscopic Methods
Dimethylanilinium Tetrakis(pentafluorophenyl)borate is characterized using essential spectroscopic techniques. Nuclear Magnetic Resonance (NMR) spectroscopy confirms structure and purity. Infrared (IR) spectroscopy identifies functional groups, while UV-Vis spectroscopy provides insights into electronic transitions and solution stability.
Analytical Techniques
Analytical methods like elemental analysis ensure compound composition and stoichiometry accuracy. X-ray crystallography offers a detailed 3D molecular structure, revealing atomic arrangements and bonding interactions crucial for understanding properties and behavior.
Data Interpretation and Structural Elucidation
Interpreting data from spectroscopic and analytical techniques involves detailed analysis. NMR spectra decipher hydrogen and carbon atom environments. IR spectra detail chemical bonding, while UV-Vis spectra elucidate electronic properties. X-ray crystallography provides precise atomic coordinates, clarifying molecular geometry. Integrating these insights provides a comprehensive understanding of Dimethylanilinium Tetrakis(pentafluorophenyl)borate’s structure and functionalities.
Physical and Chemical Properties
Physical Properties
Dimethylanilinium Tetrakis(pentafluorophenyl)borate possesses distinctive physical properties that render it invaluable across various applications. With a relatively high melting point, it exhibits stability under diverse environmental conditions. Solubility-wise, it dissolves well in organic solvents like dichloromethane and acetonitrile but remains sparingly soluble in water. These attributes are pivotal for its efficient handling and utilization in synthesis and catalytic processes.
Chemical Reactivity and Stability
Renowned for its exceptional chemical stability, Dimethylanilinium Tetrakis(pentafluorophenyl)borate owes its robustness to the structure of its borate anion. It maintains stability under ambient conditions and exhibits minimal reactivity towards moisture and air, essential factors for its safe storage and practical application. Its chemical reactivity predominantly shines in catalysis, where it acts as a potent co-catalyst in polymerization reactions, facilitating the creation of polymers with tailored properties.
Comparison with Related Compounds
Compared to other borate compounds, Dimethylanilinium Tetrakis(pentafluorophenyl)borate demonstrates superior stability and heightened reactivity. The presence of pentafluorophenyl groups enhances its electron-withdrawing capabilities, thereby amplifying its effectiveness in catalytic applications. Its intricate molecular structure enables more efficient interactions during polymerization processes, making it a preferred choice in advanced chemical syntheses.
Applications
Co-catalyst of Olefin Copolymerization
A primary application of Dimethylanilinium Tetrakis(pentafluorophenyl)borate is as a co-catalyst in olefin copolymerization. Collaborating synergistically with other catalysts, it fosters the development of copolymers endowed with enhanced mechanical and thermal properties. This capability is crucial across a spectrum of industrial sectors where specific material characteristics are paramount.
Styrene Polymerization
In the realm of styrene polymerization, this compound acts as an efficient co-catalyst, promoting the formation of polystyrene with controlled molecular weight and superior properties. Polystyrene finds widespread utility in packaging, insulation, and consumer goods, with Dimethylanilinium Tetrakis(pentafluorophenyl)borate ensuring consistent high-quality production.
Ethylene Polymerization
Utilized in ethylene polymerization, Dimethylanilinium Tetrakis(pentafluorophenyl)borate facilitates the production of polyethylene, a ubiquitous plastic. Its role as a co-catalyst enables precise control over polymerization, yielding polyethylene tailored to specific density and branching requirements. This versatility makes it indispensable in crafting diverse grades of polyethylene for applications ranging from packaging to automotive components.
Propylene Polymerization
In propylene polymerization processes, this compound enhances efficiency and control. The resulting polypropylene finds extensive use in textiles, automotive parts, and consumer products, underscoring its role in ensuring the reliable production of high-quality materials with customized properties.
Other Olefin Polymerization
Beyond ethylene and propylene, Dimethylanilinium Tetrakis(pentafluorophenyl)borate serves as a catalyst in polymerizing other olefins, offering versatility in creating a broad spectrum of polymer products. Its ability to function across various olefin polymerization processes highlights its significance in driving innovation within the chemical industry.
Copolymerization
Dimethylanilinium Tetrakis(pentafluorophenyl)borate plays a pivotal role in copolymerization, where it facilitates the synthesis of copolymers blending the distinctive properties of multiple monomers. This capability expands the repertoire of specialized materials available for diverse applications, enhancing performance characteristics beyond what single-component polymers can achieve.
Intermediates for Organic Synthesis
Beyond its catalytic roles in polymerization, Dimethylanilinium Tetrakis(pentafluorophenyl)borate serves as a crucial intermediate in organic synthesis. Its involvement in diverse chemical reactions contributes to the synthesis of complex molecules used in pharmaceuticals, agrochemicals, and fine chemicals. The compound’s stability and reactivity make it an indispensable tool for advancing chemical innovation and developing new compounds and processes.
Final Words
The synthesis and characterization of Dimethylanilinium Tetrakis(pentafluorophenyl)borate emphasize its distinctive properties and broad applications. Its exceptional stability, precise reactivity, and solubility in organic solvents position it as a crucial component in advanced chemical processes. Acting as a co-catalyst in polymerizations such as ethylene, propylene, and styrene, it facilitates the production of high-quality polymers tailored to specific needs. Moreover, its role in organic synthesis as an intermediate contributes significantly to the development of intricate molecules used in pharmaceuticals and agrochemicals. Dimethylanilinium Tetrakis(pentafluorophenyl)borate plays a pivotal role in modern chemistry, serving as an indispensable tool in both industrial applications and research endeavors.
Access Our Product Catalog and More to Discover High-Performance Chemicals Tailored to Your Business Needs