Why Ethoxy(Pentafluoro) Cyclotriphosphazene Is Important in Improving Safety Performance of Electrolyte
Why is electrolyte performance becoming so important in batteries and energy storage devices, like lithium-ion batteries? Electrolytes play a key role in improving battery efficiency, longevity, and safety. They help ions move smoothly inside the battery, which makes the battery work better and last longer. As the demand for batteries grows, there is also a greater need for safer and more reliable battery components. This is why researchers are focusing on improving electrolyte performance to meet these needs.

What is Ethoxy(Pentafluoro) Cyclotriphosphazene?
Chemical Structure and Unique Properties
Ethoxy(Pentafluoro) Cyclotriphosphazene (EPCP) is a chemical compound made up of several elements: phosphorus (P), nitrogen (N), oxygen (O), and fluorine (F). The core structure consists of a ring formed by three phosphorus atoms, each bonded to one nitrogen atom. Attached to this ring are various groups, including ethoxy (C2H5O) and pentafluoro (CF5) components. The fluorine atoms in EPCP make the compound highly reactive in terms of flame retardancy, while the ethoxy groups enhance its overall stability and solubility in various applications. This unique combination gives EPCP exceptional properties that set it apart from other compounds used in energy storage systems.
Background on Cyclotriphosphazene Compounds
Cyclotriphosphazene compounds are a class of chemicals known for their unique structural properties, such as a three-membered ring of phosphorus and nitrogen. These compounds are highly valued in materials science due to their stability, flame retardancy, and ability to form strong bonds with other materials. In the energy storage sector, cyclotriphosphazene derivatives are used to improve the performance and safety of electrolytes. Electrolytes are key components in batteries, and their stability directly impacts battery life and performance. By incorporating cyclotriphosphazene-based compounds, energy storage devices, especially lithium-ion batteries, can become safer and more efficient.
The Challenges of Electrolyte Safety in Energy Storage
Common Issues in Current Electrolyte Compositions
One of the main issues with current electrolyte compositions is their flammability. Many conventional electrolytes are highly volatile and can catch fire if the battery is exposed to high temperatures or a short circuit. Thermal instability is another concern. Electrolytes can degrade or break down when exposed to heat, which can lead to a decrease in battery performance or even dangerous situations. Leakage is also a significant issue. It can occur if the electrolyte leaks from the battery, leading to contamination and performance failure.
Risks Posed by Electrolyte Failures
When electrolytes fail, they can cause serious issues such as short circuits, fires, and reduced battery lifespan. A short circuit can occur when the electrolyte loses its ability to conduct ions properly, leading to an uncontrolled flow of electricity. This could result in overheating or even fire. Furthermore, degradation of the electrolyte affects the battery’s overall performance, reducing its capacity to hold charge and making the battery less efficient. These risks highlight the need for better, safer materials to improve electrolyte safety.
The Need for New Materials Enhance Safety Without Compromising Performance
As the demand for energy storage devices continues to rise, there is an increasing need for materials that can enhance safety without sacrificing performance. Traditional electrolyte compositions often fall short in providing both safety and high performance. Therefore, new materials like Ethoxy(Pentafluoro) Cyclotriphosphazene are being explored as alternatives to improve both safety and efficiency in energy storage devices. These new materials aim to address the issues of flammability, thermal instability, and leakage, providing a safer and more reliable option for energy storage.
How Ethoxy(Pentafluoro) Cyclotriphosphazene Improves Safety
Exploration of the Compound‘s Unique Properties
Ethoxy(Pentafluoro) Cyclotriphosphazene has several unique properties that make it an excellent choice for improving battery safety. The presence of fluorine atoms in the compound provides strong flame-retardant properties. Fluorine is known for its ability to prevent the spread of fire and inhibit combustion, making it ideal for use in energy storage applications. Additionally, the ethoxy groups enhance the compound’s stability and ability to form protective layers on electrode surfaces. These layers help prevent unwanted reactions and improve the overall performance of the battery.
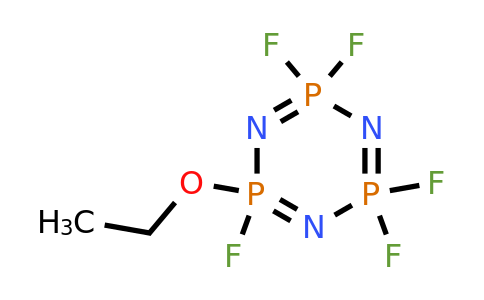
Mechanisms by Ethoxy(Pentafluoro) Cyclotriphosphazene
EPCP improves fire resistance by forming a protective barrier that prevents the electrolyte from catching fire. The fluorine components in EPCP inhibit the combustion process and reduce the risk of fires during battery operation. Additionally, EPCP’s chemical structure improves the thermal stability of electrolytes. It can withstand higher temperatures without breaking down, thus preventing the degradation of the electrolyte and increasing battery life. As a result, the overall safety of the electrolyte is significantly improved, making the battery less likely to fail in high-temperature environments.
How This Compound Helps in Reducing the Risk of Hazardous Chemical Reactions
In high-temperature environments, traditional electrolytes are prone to chemical breakdown and may release hazardous gases or chemicals. EPCP helps prevent these dangerous reactions by stabilizing the electrolyte. It forms a strong protective layer on the surface of the electrode, which minimizes the chances of unwanted chemical reactions. This not only reduces the risk of fires but also ensures that the battery continues to function properly under extreme conditions.
Why Choose UniVOOK Chemical for Ethoxy(Pentafluoro) Cyclotriphosphazene?
Flame Retardant EPCP from UniVOOK Chemical
UniVOOK Chemical produces a flame-retardant version of Ethoxy(Pentafluoro) Cyclotriphosphazene (EPCP) that is highly effective in improving the safety of energy storage devices. This compound contains both phosphorus (P) and fluorine (F) elements, which work together to provide excellent flame retardant efficiency. The combination of these two elements gives the compound superior flame resistance and thermal stability, making it an ideal choice for improving the safety of electrolytes in batteries.
High-Performance Properties of EPCP in Batteries
UniVOOK Chemical’s EPCP products are widely recognized for their flame retardant properties and their stable chemical and electrochemical behavior. In lithium-ion batteries, EPCP is used as an electrolyte flame retardant additive, where it significantly enhances the safety performance of the electrolyte. The fluorine element in EPCP helps form a solid electrolyte interphase (SEI) film on the electrode interface. This SEI film improves the compatibility between the electrolyte and the electrode, which leads to better electrochemical performance. Furthermore, EPCP helps increase the battery’s oxidation resistance and significantly improves its high-voltage cycle performance.
Applications of Flame Retardant Ethoxy(Pentafluoro) Cyclotriphosphazene
Flame retardant Ethoxy(Pentafluoro) Cyclotriphosphazene from UniVOOK Chemical is not only used in energy storage applications but also finds use in the field of electronic materials and spinning. It is commonly used as an intermediate in organic synthesis, where its flame-resistant properties add value to a wide range of products. In the electronic materials industry, EPCP is used to improve the safety and performance of components exposed to heat and electrical currents.
Applications and Benefits
How This Compound Could Revolutionize the Design of Safer Battery Systems
Ethoxy(Pentafluoro) Cyclotriphosphazene has the potential to revolutionize the design of safer battery systems, especially in electric vehicles (EVs) and portable electronics. By incorporating EPCP into the electrolyte composition, batteries can be made much safer, reducing the risk of fires and other hazards. This is particularly important for high-demand applications like EVs, where safety is a major concern. With the growing use of batteries in everyday devices, improving battery safety is critical to protect both consumers and the environment.
Potential Reduction in Safety Incidents
By improving the safety of electrolytes with compounds like EPCP, the number of safety incidents could be significantly reduced. This would lead to fewer battery failures, fires, and accidents. As a result, consumer confidence in energy storage systems would increase, encouraging further innovation and growth in the battery industry.
Possible Integration into Next-Generation Energy Storage Systems
EPCP could play a key role in next-generation energy storage systems, such as solid-state batteries. These batteries require materials that provide both high performance and safety. EPCP’s unique properties make it an excellent candidate for integration into these new technologies, helping to create batteries that are not only safer but also more efficient and durable.
Enhance Battery Safety Today with Our EPCP
Improve your battery performance and safety with Ethoxy(Pentafluoro) Cyclotriphosphazene (EPCP). This flame-retardant compound enhances thermal stability, reduces fire risks, and boosts electrochemical efficiency. Whether you’re working on electric vehicles or portable electronics, EPCP ensures safer and longer-lasting batteries. Don’t compromise on safety – choose EPCP for your next project. Contact UniVOOK Chemical now to learn more and get started!
Access Our Product Catalog and More to Discover High-Performance Chemicals Tailored to Your Business Needs




