Synthesis and Properties of Cesium Tetrakis(3,5-Dichlorophenyl)Borate
Organocesium compounds are highly valued in organic synthesis and catalysis within the realm of organometallic chemistry. These compounds, including cesium salts of organometallic anions, are known for their distinct reactivity and stability. Borates, such as tetrakis(3,5-dichlorophenyl)borate, play a pivotal role by improving reaction efficiency and selectivity in these processes. UniVOOK Chemical is a prominent supplier of high-performance Cesium Tetrakis(3,5-Dichlorophenyl)Borate(CAS No. 74127-10-9) products, widely acclaimed as catalyst materials across diverse chemical applications. Their reliability and effectiveness underscore UniVOOK’s dedication to advancing innovation and maintaining high standards in organometallic chemistry.
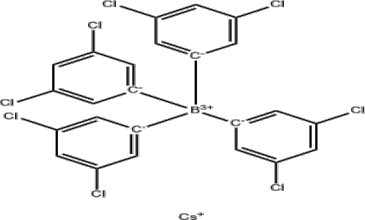
Synthesis of Cesium Tetrakis(3,5-Dichlorophenyl)Borate
Materials and Methods
1. Chemicals and Reagents
The synthesis of Cesium Tetrakis(3,5-Dichlorophenyl)Borate starts with the acquisition of high-purity chemicals and reagents. Key starting materials include cesium carbonate (Cs2CO3), 3,5-dichlorophenylboronic acid, and solvents such as tetrahydrofuran (THF) and methanol. The purity of these substances is critical to ensure the final product’s quality and yield.
2. Experimental Procedures
Experimental protocols involve precise measurements under controlled conditions. Initially, 3,5-dichlorophenylboronic acid is dissolved in a suitable solvent. Cesium carbonate is then introduced to this solution under a nitrogen atmosphere to prevent oxidation. The reaction mixture undergoes stirring and heating to a specific temperature, facilitating the formation of intermediates. Subsequent steps lead to the synthesis of the desired cesium tetrakis compound.
Synthesis Steps
1. Preparation of Intermediates
The synthesis initiates with the preparation of crucial intermediates. The reaction between 3,5-dichlorophenylboronic acid and cesium carbonate forms an intermediate borate complex. This stage typically requires prolonged stirring at elevated temperatures to ensure complete conversion of reactants into the intermediate compound.
2. Formation of the Final Compound
Following intermediate preparation, the reaction mixture undergoes further refinement and reaction stages to yield Cesium Tetrakis(3,5-Dichlorophenyl)Borate. This process involves filtration or recrystallization to remove by-products and unreacted materials. The resultant product is isolated and dried, resulting in a pure cesium tetrakis compound suitable for subsequent characterization and application in various catalytic processes.
Characterization Techniques
Spectroscopic Methods
1. NMR Spectroscopy
Nuclear Magnetic Resonance (NMR) spectroscopy serves as a primary tool for characterizing Cesium Tetrakis(3,5-Dichlorophenyl)Borate. NMR analysis provides detailed insights into the compound’s molecular structure, verifying the formation of the desired borate complex. Chemical shifts and coupling constants analyzed through NMR confirm the compound’s purity and structural integrity.
2. IR Spectroscopy
Infrared (IR) spectroscopy complements NMR by identifying functional groups within the molecule. IR spectra of Cesium Tetrakis(3,5-Dichlorophenyl)Borate display characteristic absorption bands corresponding to borate and aromatic groups. This technique corroborates the presence of key functional groups and contributes to understanding the overall molecular framework.
Analytical Techniques
1. Elemental Analysis
Elemental analysis plays a crucial role in determining Cesium Tetrakis(3,5-Dichlorophenyl)Borate’s composition. Measurement of carbon, hydrogen, chlorine, and cesium percentages confirms the synthesis’s stoichiometry accuracy. Accurate elemental analysis ensures the compound’s purity and consistency, validating its suitability for intended applications.
2. Mass Spectrometry
Mass spectrometry provides additional validation of Cesium Tetrakis(3,5-Dichlorophenyl)Borate’s molecular weight and structure. This technique ionizes the compound and measures mass-to-charge ratios of resulting fragments. Mass spectrometry data offers insights into molecular weight confirmation and aids in identifying impurities or structural variations.
Structural Analysis
X-ray Crystallography
1. Crystal Structure Determination
X-ray crystallography plays a crucial role in determining the precise crystal structure of Cesium Tetrakis(3,5-Dichlorophenyl)Borate. By directing X-rays at a crystalline sample and analyzing the resulting diffraction patterns, researchers can uncover the three-dimensional arrangement of atoms within the compound. This detailed structural information is essential for comprehending the compound’s properties and predicting its behavior in various applications.
2. Molecular Geometry and Bonding Analysis
Analyzing molecular geometry and bonding through X-ray crystallography provides valuable insights into the spatial organization and connectivity of atoms in Cesium Tetrakis(3,5-Dichlorophenyl)Borate. Understanding the angles and distances between atoms helps elucidate the compound’s stability and reactivity, guiding the design of derivatives with tailored properties. This information is pivotal for optimizing chemical reactions and developing applications that harness the compound’s structural integrity.
Physical Properties
Melting Point Determination
The melting point of Cesium Tetrakis(3,5-Dichlorophenyl)Borate is a fundamental indicator of its thermal stability. This property is determined by heating the compound under controlled conditions and recording the temperature at which it transitions from a solid to a liquid state. The precise melting point defines the temperature range suitable for the compound’s safe handling and storage, essential for its practical applications.
Solubility Characteristics
Understanding the solubility characteristics of Cesium Tetrakis(3,5-Dichlorophenyl)Borate in various solvents, such as water, methanol, and THF, is crucial for its utilization in chemical processes. Systematic testing reveals how the compound interacts with different solvents, guiding the selection of optimal conditions for synthesis and formulation. Solubility data also informs purification strategies, ensuring the compound meets stringent quality standards for industrial applications.
Chemical Reactivity and Applications
Reactivity Towards Water and Air
The reactivity of Cesium Tetrakis(3,5-Dichlorophenyl)Borate towards water and air significantly influences its storage and handling protocols. Organometallic compounds can be sensitive to moisture and oxygen, potentially leading to degradation or hazardous reactions. Evaluating the compound’s stability under varying environmental conditions involves exposing it to moisture and oxygen and monitoring any structural or property changes. This assessment is crucial for implementing effective storage practices and ensuring the compound’s integrity during use.
Potential Applications in Organic Synthesis
Cesium Tetrakis(3,5-Dichlorophenyl)Borate exhibits considerable potential in organic synthesis owing to its unique reactivity and stability. As a catalyst or reagent, it facilitates the synthesis of intricate organic molecules through processes such as cross-coupling reactions and polymerization. Its role in stabilizing reactive intermediates underscores its versatility in enhancing reaction efficiency and selectivity. Chemists leverage these properties to innovate new synthetic methodologies, thereby expanding the compound’s application scope across diverse chemical processes.
Summary
The synthesis and properties of Cesium Tetrakis(3,5-Dichlorophenyl)Borate have been meticulously examined, revealing its significant potential in organometallic chemistry. The precise structural analysis through X-ray crystallography, determination of physical properties like melting point and solubility, and evaluation of chemical reactivity underscore the compound’s stability and versatility. Its unique reactivity makes it an invaluable catalyst or reagent in various organic synthesis processes, enhancing reaction efficiency and selectivity. Comprehensive understanding of these aspects ensures safe handling, effective application, and the development of innovative synthetic methodologies. Cesium Tetrakis(3,5-Dichlorophenyl)Borate stands out as a high-performance catalyst material, driving advancements in chemical processes.
Access Our Product Catalog and More to Discover High-Performance Chemicals Tailored to Your Business Needs