CUSTOM MANUFACTURING
有限公司.jpg)
Contact Our Product Manager:
- Phone:+86 (0)21 6536 5235
- Contact: Send E-Mail
Potassium Tetrafluoroborate
| Structure Formula | 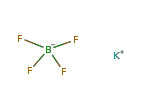 |
|---|---|
| Formula | KBF4 |
| CAS No. | 14075-53-7 |
| EINECS No. | 237-928-2 |
| Synonyms | KBF4; Potassium Tetrafluoroborate; Potassium borofluoride; |
UniVOOK Chemical supplies high performance potassium tetrafluoroborate products. As catalyst materials are widely used in chemical processes, with a unique reputation in catalyst applications.
Chemical Name: Potassium Tetrafluoroborate
Cas Number: 14075-53-7
Appearance: White Crystalline Powder
Purity(active ingredient): 98.5% min (Assay)
General Properties:
Potassium tetrafluoroborate is white powder or gel crystal, no hygroscopic, bitter taste. The relative density is 2.50g /ml and the melting point is 530℃. Slightly soluble in water and hot ethanol, insoluble in cold ethanol; It begins to decompose during melting. It can be decomposed by strong acids such as sulfuric acid to form boron trifluoride. Fluorides and borates are formed when fused with alkali metal carbonate.
Other applications of potassium tetrafluoroborate:
– Used as a flux in the production process of aluminium, titanium and boron alloys;
– Widely used in metal processing, metal surface treatment;
– Used as a filler in the manufacture of resin grinding wheels and disks to reduce the temperature during operation;
– Used as a flame retardant in cotton and man-made fibers;
– For removing corrosives from exposed lead circuit boards;
– Used as a raw material for the production of boron trifluoride and other fluoride salts
Production Process of Potassium Fluoborate:
1-Fluoroborate Potassium Pydroxide Process:
Put hydrofluoric acid and boric acid into the reaction kettle, the ratio of the two is 25:6.2 (mass ratio), the temperature does not exceed 40℃, and the reaction is 6h. The obtained fluoboric acid is sent to a neutralizing tank, where it is neutralized with potassium hydroxide at a concentration of 5 mol/L under stirring and cooling (until methyl orange is discolored). The precipitated potassium fluoborate crystals were centrifuged, washed and dried to obtain the finished potassium fluoborate.
2-Fluoroborate Potassium Carbonate Neutralization Process:
Neutralize fluoroborate acid with saturated potassium carbonate solution under agitation in a plastic coated container until methyl orange changes color. The precipitated potassium fluoborate was centrifuged, washed and dried to get the finished product.
Find more catalyst materials from UniVOOK Chemical: Lithium Fluoroborate
Under proper storage conditions, the shelf life is 12 months
For more details and safety recommendations regarding the use of the product, please consult our Material Safety Data Sheet (MSDS), or Contact With our Product Manager.



